- All living things use water to sustain their lives.
- Human beings, for example, use water for irrigation, for washing, drinking, fish farming and transportation among other uses.
- In most cases, water has some dissolved particles in it.
Such water may not be suitable for drinking, cooking, and washing. Water can be classified either as soft or hard.
What kind of water can be classified as soft water? What about hard water?
Finding out the difference between soft and hard water. What you need:
In all cases ensure that you use the same amount of soap and water and that you shake for the same period of time. What you have learned The water that formed lather with soap easily is said to be soft water. The water that did not form lather easily is said to be hard water. Water from sources such as borehole, sea or ocean, lake, well, tap and river are hard water. They have minerals dissolved in them which makes them hard. Rainwater and sometimes spring water is known to be soft water.
The source of water determines whether the water is soft or hard. Some sources may contain salts while others may not contain salts.
As we have learned, hard water does not easily lather with soap. Obviously, this is one of the disadvantages of hard water. When using hard water for washing, a lot of soap is used compared to when soft water is used.
The other disadvantages of hard water are as follows:
Finding out the effect of water hardness on the cleaning ability of soap. What you need :
Scum that forms when hard water is mixed with soap affects the cleaning power of soap. It reduces the effects of soap therefore a lot of soap has to be used and clothes need much more rubbing to remove dirt or stains.
Clothes cleaned in hard water might last for a shorter period since they have to be rubbed harder to remove the dirt or stain.
They also last for a short time because the minerals in hard water are rough on clothes.
When bathing, more soap is used if the water is hard than if the water is soft.
Finding out what happens when hard water is heated
What you need
Why is the diameter of the spout of the old kettle narrower than the spout of the new one?
Fur is a bad conductor of heat. It therefore reduces the amount of heat reaching the water. It takes much longer to heat the water leading to wastage of fuel.

It is possible for hard water to become soft. This is done by removing the mineral salts that cause water hardness.
The process of removing mineral salts from hard water is called softening. Hard water can be softened in two ways:
Softening hard water by boiling What you need:
Lather is more easily formed in boiled water than in unboiled water.
Boiling changed hard water into soft water.
Hard water can be softened by boiling it.
However, not all hard water can be made soft through boiling.
Hard water which can be changed into soft water through boiling is said to show temporary hardness.
It is therefore referred to as temporary hard water.
Water that cannot be made soft by boiling shows permanent hardness.
It is known as permanent hard water. Permanent hardness in water can only be removed by the use of chemicals.
Below are some of the new words you have learnt in this topic.

1. Water can be said to be ________________ or _________________.
2. Name two sources of
a) hard water.
b) soft water.
3. Which of the following is NOT TRUE about hard water?
A. Lathers very easily with soap.
B. Forms scum with soap.
C. It has mineral salts.
D. Forms milky appearance with soap.
4. Which of the following is the name given to the dark-grey coating that forms in kettles and pans after boiling water?
A. Boiler scale
B. Scum
C. Fur
D. Lather
5. Temporary hardness in water can be removed by _________________.
6. Which of the following is NOT true about hard water?
A. Damages hot water pipes.
B. Uses less soap.
C. Wastes soap.
D. Damages clothes.
7. How does hard water affect the following?
(a) Clothes (b) Pipes (c) Pans
8. What is scum and how is it formed?
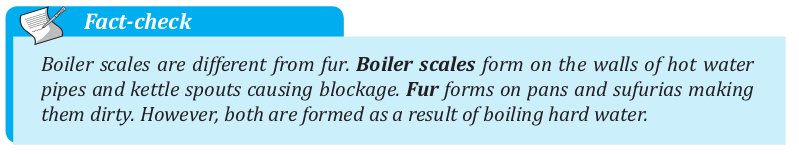
When hard water is heated, it forms substances that cannot dissolve in water.
When the heated water is passed through water pipes or through kettles, these substances are deposited on the pipes or spouts of kettles.
These substances are called boiler scale. Scales are the cause of pipes getting blocked.
Also, when you boil water on a sufuria or pan, you notice the inside of the sufuria or pan changes colour to pale-grey.
This is as a result of the formation of fur on the sufuria or pan.
Standard 8
1. Pupils in Standard VII at Omeri Primary School were given different volumes of four clear samples of water labeled W, X, Y and Z. They boiled each sample in turn to evaporate all the water and then collected and weighed the solids that had remained. Their results were as shown below.
|
Water sample |
Volume of water evaporated (cm3) |
Amount of solid collected (g) |
|
W X Y Z |
50 100 200 300 |
2.5 2.0 5.0 3.0 |
Which one of the four samples would form lather with soap MOST EASILY?
A. W B. X C. Y D. Z